Ph.D. - Applied and Engineering Physics
Cornell University (Ithaca, NY)
Jason Slinker
Associate Department Head of Physics
Professor – Physics
Mentor, Society of Physics Students
Affiliated Faculty, UTD Department Materials Science and Engineering & Department of Chemistry
We create mixed conductor optoelectronic devices for energy efficiency and novel biosensors for disease diagnostics and laboratory assays.
Professional Preparation
M.S. - Applied and Engineering Physics
Cornell University (Ithaca, NY)
Cornell University (Ithaca, NY)
B.S. - Physics, Chemistry and Math (Triple major, GPA: 4.0)
Southern Nazarene University
Southern Nazarene University
Research Areas
Optoelectronics from Mixed Conductors
Ionic and electronic conductivity are leveraged to bring about high efficiency light emitting devices and solar cells with simple, low-cost architectures.Electrochemistry of Perovskites
Leveraging hydrofluoroethers as a nondestructive electrolyte for electrochemical interrogation and doping of a variety of perovskite optoelectronic materials.Electrochemical Sensors of Protein and Drug Activity
Electrochemical DNA devices are used to sense anticancer drug and protein activity that disrupts the structure of the double helix.Bioinspired Molecular Wires
Utilizing DNA-inspired molecular nanowire devices with high fidelity and reproducibility.Publications
Isotope-Edited Variable Temperature Infrared Spectroscopy for Measuring Transition Temperatures of Single A-T Watson–Crick Base Pairs in DNA Duplexes 2024 - Journal Article
Highly Efficient Quasi 2D Blue Perovskite Electroluminescence Leveraging a Dual Ligand Composition 2023 - Journal Article
CdS/CdSe/CdS Spherical Quantum Wells with Near-Unity Biexciton Quantum Yield for Light-Emitting-Device Applications 2023 - Journal Article
Molecular wrench activity of DNA helicases: Keys to modulation of rapid kinetics in DNA repair 2023 - Journal Article
The Synergetic Ionic and Electronic Features of MAPbI3 Perovskite Films Revealed by Electrochemical Impedance Spectroscopy 2023 - Journal Article
Revealing the Low-Temperature Interplay of Electronic, Ionic, and Optical Effects in Perovskite Electroluminescent Devices 2023 - Journal Article
Dielectric constants and double-layer formation in a perovskite thin film revealed by electrochemical impedance spectroscopy 2023 - Journal Article
Awards
Jonathan F. Reichert & Barbara Wolff-Reichert Award for Excellence in Advanced Laboratory Instruction - American Physical Society [2025]
Provost's Award for Excellence in Undergraduate Research Mentoring - UT Dallas [2022]
Hyer Award, Undergraduate Research Mentoring - American Physical Society, Texas Section [2019]
Institutional Improvement Award - The University of Texas at Dallas [2018]
Hyer Award, Graduate Research Mentoring - American Physical Society, Texas Section [2014]
Regent's Outstanding Teaching Award - University of Texas System [2014]
Outstanding Alumni Award - Southern Nazarene University [2013]
Appointments
Professor
The University of Texas at Dallas [2024–Present]
Physics Associate Department Head Affiliated Faculty, Materials Science and Engineering; Mentor, UTD Society of Physics Students International Advisory Board ChemPlusChem
The University of Texas at Dallas [2024–Present]
Physics Associate Department Head Affiliated Faculty, Materials Science and Engineering; Mentor, UTD Society of Physics Students International Advisory Board ChemPlusChem
Associate Professor
The University of Texas at Dallas [2016–2024]
Physics Undergraduate Program Head 2022 UTD Provost's Award for Excellence in Undergraduate Research Mentoring 2019 Texas Section American Physical Society Hyer Award (with Nolan King)
The University of Texas at Dallas [2016–2024]
Physics Undergraduate Program Head 2022 UTD Provost's Award for Excellence in Undergraduate Research Mentoring 2019 Texas Section American Physical Society Hyer Award (with Nolan King)
Assistant Professor
The University of Texas at Dallas [2010–2016]
-2014 University of Texas System Regent's Outstanding Teacher Award -2014 Texas Section American Physical Society Hyer Award (with Marc McWilliams)
The University of Texas at Dallas [2010–2016]
-2014 University of Texas System Regent's Outstanding Teacher Award -2014 Texas Section American Physical Society Hyer Award (with Marc McWilliams)
Postdoctoral Scholar
California Institute of Technology [2007–2010]
Advisor: Jackie K. Barton Electrochemical protein detection with DNA-modified electrodes Ruth L. Kirschstein National Research Service Award (NRSA) postdoctoral fellowship
California Institute of Technology [2007–2010]
Advisor: Jackie K. Barton Electrochemical protein detection with DNA-modified electrodes Ruth L. Kirschstein National Research Service Award (NRSA) postdoctoral fellowship
Graduate Research Assistant
Cornell University [2002–2007]
Advisor: George G. Malliaras Understanding and improving light emitting devices based on ionic transition metal complexes (iTMCs) National Science Foundation Graduate Research Fellowship
Cornell University [2002–2007]
Advisor: George G. Malliaras Understanding and improving light emitting devices based on ionic transition metal complexes (iTMCs) National Science Foundation Graduate Research Fellowship
Visiting Scientist
University of Cambridge (England) [2005–2005]
Advisor: Richard H. Friend Used Raman spectroscopy to study the degradation of iTMC devices by tracking the in situ formation and spatial location of luminescence-quenching compounds.
University of Cambridge (England) [2005–2005]
Advisor: Richard H. Friend Used Raman spectroscopy to study the degradation of iTMC devices by tracking the in situ formation and spatial location of luminescence-quenching compounds.
Additional Information
Patents
3D-0D Perovskite Light-Emitting Electrochemical Cells. J. D. Slinker, A. Mishra, R. Bose, A. Malko. US Patent No. 12,490,576 (2025).Electrospun light-emitting fibers. J. Moran-Mirabal, H. G. Craighead, G. G. Malliaras, H. D. Abruña, and J. D. Slinker. US Patent No. 8,541,940 (2013).
Electrospun light-emitting nanofibers. J. Moran-Mirabal, H. G. Craighead, G. G. Malliaras, H. D. Abruña, and J. D. Slinker. US Patent No. 8,106,580 (2012).
Cascaded light emitting devices based on mixed conductor electroluminescence. G. G. Malliaras, K. Mori, J. D. Slinker, D. A. Bernards, and H. D. Abruña. US Patent No. 8,063,566 (2011).
Cascaded light emitting devices based on mixed conductor electroluminescence. G. G. Malliaras, K. Mori, J. D. Slinker, D. A. Bernards, and H. D. Abruña. US Patent No. 7,755,275 (2010).
News Articles
Leveraging a Stable Perovskite Composite to Satisfy Blue Electroluminescence Standards
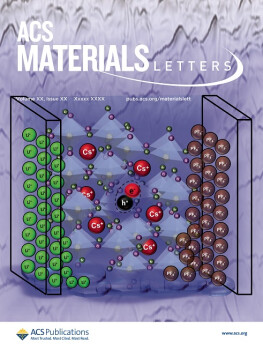 Differential ion motion in mixed halide blue perovskite light-emitting electrochemical cells is produced with a LiPF6 salt additive, which dissociates to form electrical double layers at the electrodes and preserves the underlying perovskite structure. This combination, together with supporting electrolyte polymers, produced stable pure blue electroluminescence surpassing standard benchmarks. The background shows an edge glow rendering of the atomic force microscopy image of a blended thin film used in this study.
Differential ion motion in mixed halide blue perovskite light-emitting electrochemical cells is produced with a LiPF6 salt additive, which dissociates to form electrical double layers at the electrodes and preserves the underlying perovskite structure. This combination, together with supporting electrolyte polymers, produced stable pure blue electroluminescence surpassing standard benchmarks. The background shows an edge glow rendering of the atomic force microscopy image of a blended thin film used in this study.
Pure Blue Electroluminescence: Pure Blue Electroluminescence by Differentiated Ion Motion in a Single Layer Perovskite Device
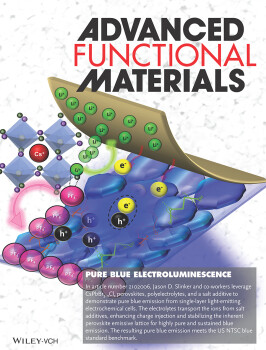 In article number 2102006, Jason D. Slinker and co-workers leverage CsPbBr3−xClx perovskites, polyelectrolytes, and a salt additive to demonstrate pure blue emission from single-layer light-emitting electrochemical cells. The electrolytes transport the ions from salt additives, enhancing charge injection and stabilizing the inherent perovskite emissive lattice for highly pure and sustained blue emission. The resulting pure blue emission meets the US NTSC blue standard benchmark.
In article number 2102006, Jason D. Slinker and co-workers leverage CsPbBr3−xClx perovskites, polyelectrolytes, and a salt additive to demonstrate pure blue emission from single-layer light-emitting electrochemical cells. The electrolytes transport the ions from salt additives, enhancing charge injection and stabilizing the inherent perovskite emissive lattice for highly pure and sustained blue emission. The resulting pure blue emission meets the US NTSC blue standard benchmark.
Bright Single-Layer Perovskite Host–Ionic Guest Light-Emitting Electrochemical Cells
 To achieve high performance in a simple single-layer device, a CsPbBr3 perovskite host and a novel ionic iridium complex guest were utilized along with a polyelectrolyte to demonstrate efficient light-emitting electrochemical cells. Perovskites are excellent solution-processable conductive materials, and iridium complexes continue to be of great interest as highly luminophoric materials. In light-emitting electrochemical cells, the application of a bias induces ionic redistribution that facilitates electron and hole injection and subsequent light emission.
To achieve high performance in a simple single-layer device, a CsPbBr3 perovskite host and a novel ionic iridium complex guest were utilized along with a polyelectrolyte to demonstrate efficient light-emitting electrochemical cells. Perovskites are excellent solution-processable conductive materials, and iridium complexes continue to be of great interest as highly luminophoric materials. In light-emitting electrochemical cells, the application of a bias induces ionic redistribution that facilitates electron and hole injection and subsequent light emission.
Perovskite Light‐Emitting Electrochemical Cells: Enhanced Operational Stability of Perovskite Light‐Emitting Electrochemical Cells Leveraging Ionic Additives
 Perovskite light‐emitting electrochemical cells (PeLECs) utilize ionic redistribution to emit light efficiently from single layer devices. As demonstrated by Jason D. Slinker and co‐workers in article number 2000226, PeLECs show 100 h operation in excess of 800 cd m−2 and extrapolated lifetimes of 6700 h at 100 cd m−2 with an optimal concentration of a lithium salt additive. Electrochemical impedance spectroscopy reveals lithium additives enhance efficiency through improved electrical double layer formation.
Perovskite light‐emitting electrochemical cells (PeLECs) utilize ionic redistribution to emit light efficiently from single layer devices. As demonstrated by Jason D. Slinker and co‐workers in article number 2000226, PeLECs show 100 h operation in excess of 800 cd m−2 and extrapolated lifetimes of 6700 h at 100 cd m−2 with an optimal concentration of a lithium salt additive. Electrochemical impedance spectroscopy reveals lithium additives enhance efficiency through improved electrical double layer formation.
Bright and Effectual Perovskite Light-Emitting Electrochemical Cells Leveraging Ionic Additives
 We leveraged a poly(ethylene oxide) electrolyte and a lithium salt in CsPbBr3 thin films to produce ~15000 cd/m2 performance in perovskite light-emitting electrochemical cells. We find that lithium salt addition reduces the occurrence of voids, charge traps, and pinholes and increases grain size and packing density. View the article.
We leveraged a poly(ethylene oxide) electrolyte and a lithium salt in CsPbBr3 thin films to produce ~15000 cd/m2 performance in perovskite light-emitting electrochemical cells. We find that lithium salt addition reduces the occurrence of voids, charge traps, and pinholes and increases grain size and packing density. View the article.